
Studies conducted by the
New England Journal of Medicine show that coronaviruses remain viable on plastics for 72 hours, stainless steel for 48 hours, cardboard for 24 hours and copper for 4 hours. Residues remaining on surfaces can also sustain the viability of bacteria. This highlights the significance of cleaning and verifying the cleanliness of your weighing machinery and work areas.
It is important to make sure your equipment is appropriate for your environment. The legal requirements for hygienic design of food machinery, set out by the
Health and Safety Executive (HSE) stipulate that machines must be designed and constructed so that:
- they can be cleaned before each use and can be easily dismantled for cleaning where necessary.
- materials coming into contact with food satisfy certain conditions as to suitability and surfaces in contact with food are smooth, have minimum edges, recesses etc.
- cleaning or disinfecting chemicals can be completely discharged from the machine.
- insects, organic matter etc. cannot accumulate in areas that cannot be cleaned.
- machine lubricants etc cannot come into contact with foods.
The
EHEDG (European Hygienic Engineering and Design Group) Hygienic Design Principles 2018 offers guidance on the design and maintenance of food production systems. The EHEDG guides cover all aspects of food production and manufacturing infrastructure so hygienic design principles can be applied to reduce the risk of contamination and not adversely affect food safety.
Advances in technology now mean that equipment designed for use within the food industry have features including touchless, smart sensors and hygienic gas-lift platforms for thorough cleaning.
Cleaning Considerations:
- Wash your hands before and after you clean or use your equipment
- Check manufacturers’ guidance to ensure the use of appropriate disinfectants and never use of harsh chemicals. Appropriate biocides for inactivating viruses should have BS EN 14476 certification. BS EN 14476 includes rotaviruses which are encapsulated viruses, of which COVID 19 is part.
- Is the surrounding environment clean to limit cross-contamination?
- Unless it expressly says it is okay to do so in guidance, do not spray any cleaning solutions directly on the equipment. Spray the solution onto a cleaning cloth and wipe it with that.
- Stainless steel or plastic weighing equipment with Ingress Protection to IP65, IP66, IP67 or IP69K can be sprayed onto directly with appropriate solutions – but check the manufacturer guidance to verify it is safe to do so.
- When removing or dismantling any parts of the equipment to clean separately pay close attention to any parts where dirt could be caught.
- Where are the high touch points on the equipment, such as the keypad? Are these being sanitised at regular intervals?
- Is regular cleaning of your equipment incorporated into your HACCP plan?
Verifying hygiene and cleaning procedures
To demonstrate the effectiveness of cleaning many companies use a hygiene monitoring system that helps verify that their procedures are working
. The most popular hygiene monitoring systems use Adenosine Triphosphate (ATP) technology to give an indication of cleanliness on a surface on in clean-in-place (CIP) rinse water.
ATP Hygiene monitoring works by using a swab to sample an area, for example, a keypad on a checkweighing balance. The swab is then activated and then placed inside a luminometer. The luminometer analyses the swab and gives a reading back as a numerical value known as a relative light unit (RLU). You can then use these results to determine if the area needs to be recleaned.
Benefits of using ATP solutions:
- Demonstrates due diligence
- Helps reduce the risk of cross-contamination due to poor cleaning
- Tracks cleaning efforts and verifies they have been effective
- Helps protect your reputation
How ATP hygiene technology works
ATP is an energy molecule found in all plant, animal and microbial cells and it fuels metabolic processes. All organic matter (living or once-living) contains ATP, including food, bacteria, mould, and other microorganisms.
ATP testing devices contain a natural enzyme found in fireflies. This enzyme, called luciferase, produces simple bioluminescence (light-producing) reaction when it comes into contact with ATP. Using bioluminescence technology, ATP luminometers can measure extremely low levels of ATP collected with testing devices.
Measuring the amount of bioluminescence from an ATP reaction on an activated swab provides an excellent indication of surface cleanliness because the quantity of light generated by the reaction is directly proportional to the amount of ATP present in the sample.
Important ATP Product Capability Information:
- ATP meters do not detect viruses
- Performing ATP Cleaning Verification does not guarantee removal of viruses on surfaces
- ATP Cleaning Verification should be used in conjunction with Public Health and NHS recommendations and guidelines
Gem Scientific are independent hygiene verification specialists and our team are on hand to talk you through the different options available to find the right system for you.
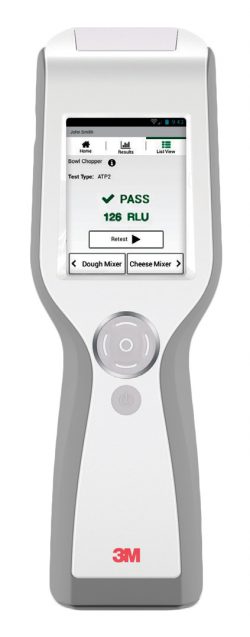 |
- Audit ready system for BRC or retailer inspections
- Record all your HACCP results on the 3M Clean-Trace LM1 Luminometer
- Colour touch screen works seamlessly even when you’re wearing gloves
- Watch lists pinpoint problems at a glance to enforce corrective action steps
- Easy-to-use dashboard immediately turns data into actionable information
|
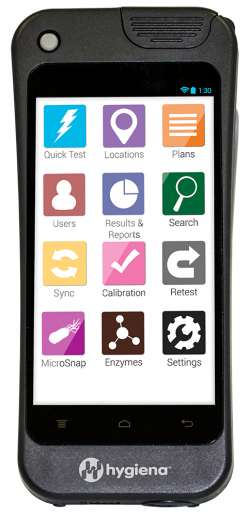 |
- Functions like a smartphone with intuitive smart navigation
- Safe and responsive touchscreen
- Customizable to your operation – Facility type, Language, Data fields, etc
- Secure cloud-based data management
- Advanced photodiode technology
- USB or Wi-Fi sync capability
- Responsive 5-inch shatter-proof touchscreen
- Train remote teams with built-in screen sharing capability
|

Here are some further articles about the benefits of using ATP monitoring
05/06/2020: edited to expand on the first paragraph to include different time scales per surface and to include links to the EHEDG Hygienic Design Principles 2018





 Studies conducted by the
Studies conducted by the 

